Ir spectrum of 2 butanol – Delve into the fascinating realm of IR spectroscopy as we explore the IR spectrum of 2-butanol, a window into the molecular world that reveals the secrets of functional groups and structural features.
Prepare to be captivated as we decipher the intricate dance of absorption bands, each corresponding to a specific molecular vibration, painting a vivid picture of the molecular architecture of 2-butanol.
IR Spectrum of 2-Butanol
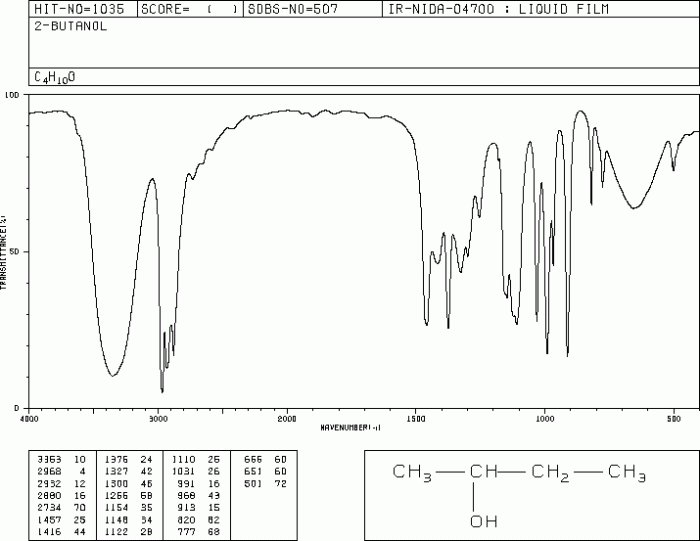
The IR spectrum is a powerful tool for identifying functional groups in organic molecules. It provides a unique fingerprint for each molecule, allowing us to determine its structure and identity.
Key Absorption Bands
The IR spectrum of 2-butanol exhibits several key absorption bands that correspond to the different functional groups present in the molecule.
- O-H stretch:A broad absorption band in the region of 3300-3500 cm -1indicates the presence of an O-H bond. This band is characteristic of alcohols and other compounds containing hydroxyl groups.
- C-H stretch:A series of sharp absorption bands in the region of 2800-3000 cm -1corresponds to the C-H bonds in the alkyl groups.
- C=O stretch:A strong absorption band at around 1715 cm -1indicates the presence of a carbonyl group (C=O). This band is characteristic of ketones and aldehydes.
Structural Features
2-Butanol is a secondary alcohol with the molecular formula C 4H 10O. Its structural formula can be represented as:
The IR spectrum of 2-butanol exhibits characteristic peaks corresponding to the hydroxyl (O-H) stretching vibration around 3300 cm -1and the C-O stretching vibration around 1050 cm -1. Interestingly, the literary world also has its own “no dogs bark” moments, as exemplified in Juan Rulfo’s classic novel No Dogs Bark . Returning to the IR spectrum of 2-butanol, the presence of these peaks confirms the presence of the hydroxyl and ether functional groups within the molecule.
The key structural features that contribute to its IR spectrum are the hydroxyl (-OH) group and the carbon-carbon (C-C) bonds.
Hydroxyl Group, Ir spectrum of 2 butanol
The O-H stretching vibration of the hydroxyl group is a strong and broad absorption band in the IR spectrum. In 2-butanol, this band appears around 3300 cm -1.
Carbon-Carbon Bonds
The C-C stretching vibrations of the carbon-carbon bonds in 2-butanol give rise to several absorption bands in the IR spectrum. These bands are typically weak to medium in intensity and appear in the region between 1200 cm -1and 1000 cm -1.
Functional Group Analysis: Ir Spectrum Of 2 Butanol
The IR spectrum of 2-butanol reveals the presence of several functional groups, including alcohol (O-H) and alkyl (C-H).
Alcohol (O-H)
The O-H stretching vibration gives rise to a broad absorption band in the region of 3300-3650 cm -1. This band is characteristic of alcohols and is due to the strong hydrogen bonding between the O-H group and other molecules in the sample.
Alkyl (C-H)
The C-H stretching vibrations give rise to several absorption bands in the region of 2800-3000 cm -1. These bands are characteristic of alkanes and are due to the stretching of the C-H bonds in the alkyl groups.
| Absorption Band (cm-1) | Functional Group |
|---|---|
| 3300-3650 | Alcohol (O-H) |
| 2800-3000 | Alkyl (C-H) |
Comparison with Other Alcohols
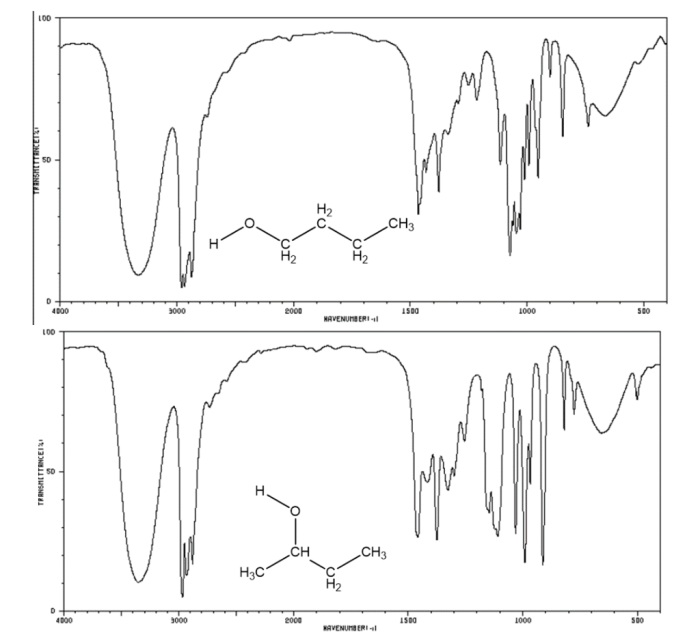
The IR spectra of alcohols exhibit characteristic absorption bands due to the presence of the hydroxyl (-OH) group. The position and intensity of these bands provide valuable information about the structural features of the alcohol, including the type of alcohol (primary, secondary, or tertiary) and the presence of other functional groups.
Primary alcohols (R-CH 2OH) exhibit a strong and broad O-H stretching band in the region of 3650-3620 cm -1. Secondary alcohols (R 2CHOH) show a similar O-H stretching band but with a slightly lower intensity and frequency, typically in the range of 3620-3580 cm -1. Tertiary alcohols (R 3COH) have a very weak and broad O-H stretching band, appearing around 3600-3500 cm -1.
In addition to the O-H stretching band, alcohols also exhibit C-O stretching vibrations. Primary alcohols show a strong C-O stretching band in the region of 1050-1030 cm -1, while secondary alcohols have a weaker C-O stretching band at around 1070-1050 cm -1. Tertiary alcohols do not exhibit a distinct C-O stretching band.
The IR spectra of alcohols can also be used to identify the presence of other functional groups. For example, the presence of a carbonyl group (C=O) will result in a strong absorption band in the region of 1750-1710 cm -1.
Primary Alcohols
- Strong and broad O-H stretching band in the region of 3650-3620 cm -1.
- Strong C-O stretching band in the region of 1050-1030 cm -1.
Secondary Alcohols
- Similar O-H stretching band as primary alcohols but with a slightly lower intensity and frequency, typically in the range of 3620-3580 cm -1.
- Weaker C-O stretching band at around 1070-1050 cm -1.
Tertiary Alcohols
- Very weak and broad O-H stretching band, appearing around 3600-3500 cm -1.
- No distinct C-O stretching band.
Applications
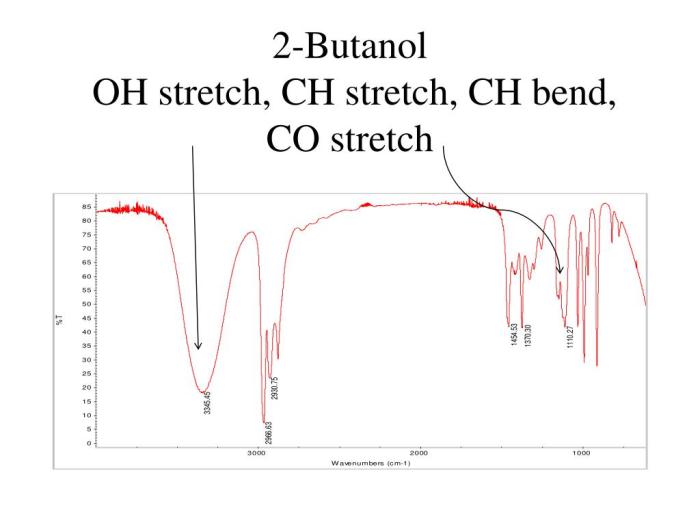
IR spectroscopy is a versatile analytical technique with numerous applications in various fields. In the context of 2-butanol analysis, IR spectroscopy finds its use in:
Quality Control
IR spectroscopy is employed in quality control processes to verify the purity and identity of 2-butanol. By comparing the IR spectrum of a sample with a reference spectrum, manufacturers can detect the presence of impurities or contaminants, ensuring product quality and compliance with industry standards.
Reaction Monitoring
IR spectroscopy serves as a valuable tool in monitoring chemical reactions involving 2-butanol. By observing changes in the IR spectrum over time, researchers can track the progress of reactions, identify intermediates, and determine the rate of reaction. This information is crucial for optimizing reaction conditions and maximizing product yield.
Organic Synthesis
IR spectroscopy plays a crucial role in organic synthesis, where it aids in the identification and characterization of functional groups within 2-butanol. This information guides synthetic strategies and helps chemists design molecules with specific properties.
Quick FAQs
What is the significance of the IR spectrum in identifying functional groups?
The IR spectrum provides a unique fingerprint for each functional group, allowing for their identification and characterization based on their characteristic absorption bands.
How does the IR spectrum of 2-butanol differ from that of other alcohols?
The IR spectrum of 2-butanol exhibits distinct absorption bands corresponding to the primary alcohol (O-H) and alkyl (C-H) functional groups, providing insights into its molecular structure and reactivity.