Identify the geometry about interior atoms in ch3nh2. – In the realm of chemistry, understanding the molecular geometry of compounds is crucial for unraveling their properties and reactivity. Among these, CH3NH2 stands out as a molecule with intriguing structural features that have captivated the attention of researchers. This article delves into the intricate details of the geometry about interior atoms in CH3NH2, shedding light on its molecular architecture through the lenses of theory and experiment.
The molecular geometry of CH3NH2 is shaped by the interplay of bonding arrangements, hybridization, and the presence of lone pairs of electrons. Nitrogen, the central atom in CH3NH2, exhibits sp3 hybridization, giving rise to a tetrahedral electron pair geometry. However, the presence of a lone pair on the nitrogen atom disrupts the tetrahedral symmetry, resulting in a distorted tetrahedral molecular geometry.
This distortion has profound implications for the molecule’s overall structure and properties.
Geometry of Interior Atoms in CH3NH2
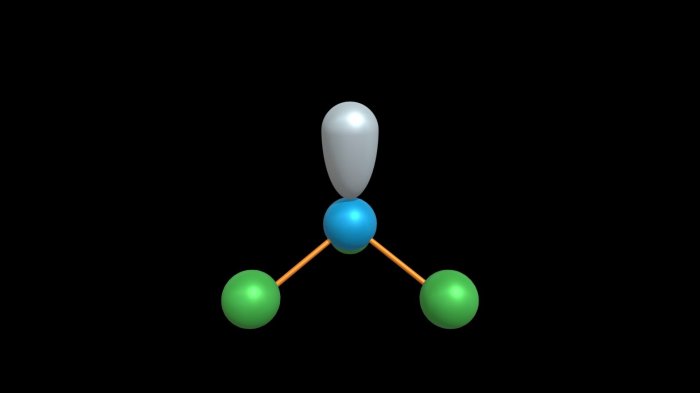
The molecular geometry of CH3NH2, methylamine, is determined by the bonding arrangement and hybridization of the interior atoms, nitrogen (N) and hydrogen (H). The N atom is the central atom and is bonded to three H atoms and one C atom.
The H atoms are arranged in a trigonal pyramidal geometry around the N atom, with the C atom occupying the fourth position.
Bonding Arrangement and Hybridization of N and H
The N atom in CH3NH2 has three valence electrons, and each H atom contributes one valence electron. This gives a total of eight valence electrons, which are used to form four covalent bonds. The N atom undergoes sp 3hybridization, which means that it mixes its 2s and three 2p orbitals to form four equivalent hybrid orbitals.
These hybrid orbitals are then used to form sigma bonds with the three H atoms and one C atom.
Lone Pair of Electrons on the N Atom, Identify the geometry about interior atoms in ch3nh2.
In addition to the four bonding pairs of electrons, the N atom also has one lone pair of electrons. This lone pair occupies one of the sp 3hybrid orbitals and is not involved in bonding. The presence of the lone pair affects the molecular geometry by repelling the bonding pairs of electrons, causing the H atoms to be pushed away from the lone pair and towards each other.
This results in the trigonal pyramidal geometry of the H atoms.
Molecular Orbital Theory and Geometry
Molecular orbital theory can be used to explain the geometry of CH3NH2. The molecular orbitals are formed by the linear combination of atomic orbitals. The atomic orbitals of the N and H atoms interact to form bonding and antibonding molecular orbitals.
The bonding molecular orbitals are lower in energy than the atomic orbitals, and the antibonding molecular orbitals are higher in energy.
Formation of Molecular Orbitals
The four sp 3hybrid orbitals on the N atom interact with the 1s orbitals of the three H atoms to form four bonding molecular orbitals. These bonding molecular orbitals are designated as σ(N-H). The lone pair of electrons on the N atom occupies a non-bonding molecular orbital, which is designated as n(N).
The antibonding molecular orbitals, designated as σ*(N-H), are not occupied.
Energy Levels and Shapes of Molecular Orbitals
The energy levels of the molecular orbitals determine the stability of the molecule. The bonding molecular orbitals are lower in energy than the antibonding molecular orbitals, so the electrons occupy the bonding molecular orbitals. The shapes of the molecular orbitals determine the geometry of the molecule.
The σ(N-H) bonding molecular orbitals have a symmetrical distribution of electron density around the N-H bond axis. This results in the trigonal pyramidal geometry of the H atoms.
Spectroscopic Techniques for Geometry Determination: Identify The Geometry About Interior Atoms In Ch3nh2.
Various spectroscopic techniques can be used to determine the geometry of CH3NH 2. These techniques include:
- Infrared (IR) spectroscopy
- Nuclear magnetic resonance (NMR) spectroscopy
- Microwave spectroscopy
- Electron diffraction
- X-ray crystallography
Each of these techniques provides information about the molecular geometry by measuring different properties of the molecule. For example, IR spectroscopy measures the vibrational frequencies of the molecule, which can be used to determine the bond lengths and angles. NMR spectroscopy measures the magnetic properties of the molecule, which can be used to determine the connectivity of the atoms.
Computational Methods for Geometry Optimization

Computational methods can be used to optimize the geometry of CH3NH2. These methods use mathematical algorithms to find the geometry that minimizes the energy of the molecule. The most common computational method for geometry optimization is the Hartree-Fock method. The Hartree-Fock method uses a self-consistent field approach to solve the Schrödinger equation for the molecule.
Different Computational Approaches
There are different computational approaches that can be used for geometry optimization, including:
- Hartree-Fock (HF)
- Density functional theory (DFT)
- Second-order Møller-Plesset perturbation theory (MP2)
Each of these approaches has its own advantages and disadvantages. The HF method is the simplest and most computationally efficient, but it can be less accurate than DFT and MP2. DFT is more accurate than HF, but it is also more computationally expensive.
MP2 is the most accurate of the three methods, but it is also the most computationally expensive.
Role of Basis Sets and Functionals
The accuracy of computational methods for geometry optimization depends on the choice of basis set and functional. The basis set is a set of functions that are used to represent the molecular orbitals. The functional is a mathematical expression that is used to calculate the energy of the molecule.
There are many different basis sets and functionals available, and the choice of basis set and functional can affect the accuracy of the geometry optimization.
Comparison with Other Amine Derivatives
The geometry of CH3NH2 is similar to that of other amine derivatives. Amine derivatives have a nitrogen atom that is bonded to three hydrogen atoms and one carbon atom. The geometry of the nitrogen atom is typically trigonal pyramidal, with the hydrogen atoms arranged in a trigonal pyramidal geometry around the nitrogen atom.
The C-N bond length and the N-H bond lengths are also similar in different amine derivatives.
Effects of Different Substituents
The geometry of amine derivatives can be affected by the presence of different substituents on the nitrogen atom. For example, the presence of a methyl group on the nitrogen atom can cause the nitrogen atom to adopt a more planar geometry.
The presence of a bulky substituent, such as a tert-butyl group, can cause the nitrogen atom to adopt a more tetrahedral geometry.
Insights into Electronic Structure and Bonding
The comparison of the geometry of CH3NH2 to other amine derivatives provides insights into the electronic structure and bonding of amine compounds. The geometry of the nitrogen atom is determined by the hybridization of the nitrogen atom and the presence of lone pairs of electrons.
The effects of different substituents on the geometry of the nitrogen atom can be explained by the electronic effects of the substituents.
FAQ Insights
What is the hybridization of the nitrogen atom in CH3NH2?
sp3
How does the lone pair on the nitrogen atom affect the molecular geometry?
It distorts the tetrahedral electron pair geometry, resulting in a distorted tetrahedral molecular geometry.
What spectroscopic techniques can be used to determine the geometry of CH3NH2?
Infrared spectroscopy, Raman spectroscopy, and microwave spectroscopy