Embarking on a scientific odyssey, we delve into the atomic arrangement of a glass pitcher, a material that has captivated scientists and artisans alike for centuries. This intricate atomic structure imparts unique properties to glass, making it an indispensable material in countless applications.
From its unparalleled transparency to its remarkable strength, the atomic arrangement of glass plays a pivotal role in shaping its physical characteristics. Understanding this arrangement empowers us to harness the full potential of glass and unlock its limitless possibilities.
Atomic Structure of Glass
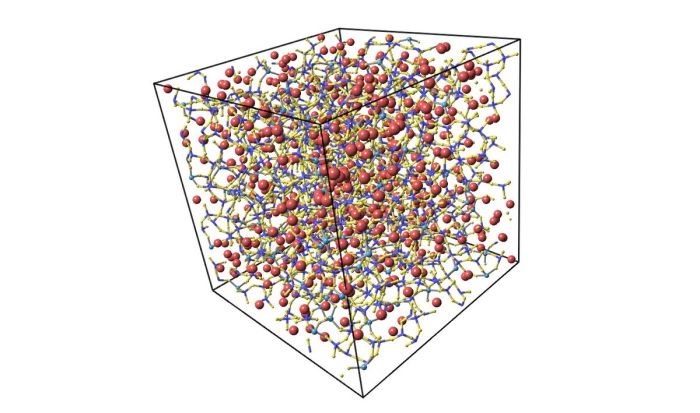
Unlike crystalline materials, glass does not have a regular, repeating arrangement of atoms. Instead, the atoms in glass are arranged in a random, disordered manner. This is because glass is formed when a liquid is cooled rapidly, preventing the atoms from arranging themselves in a crystalline structure.
Types of Bonds in Glass
The atoms in glass are held together by a variety of bonds, including:
- Covalent bonds:These are strong bonds formed when two atoms share electrons. Covalent bonds are the strongest type of bond found in glass.
- Ionic bonds:These are bonds formed when one atom transfers electrons to another atom. Ionic bonds are weaker than covalent bonds, but they are still strong enough to hold the atoms in glass together.
- Metallic bonds:These are bonds formed between metal atoms. Metallic bonds are weak, but they are still strong enough to hold the atoms in glass together.
The type of bond that is formed between two atoms in glass depends on the electronegativity of the atoms. Electronegativity is a measure of how strongly an atom attracts electrons. The more electronegative an atom is, the more strongly it attracts electrons.In
general, atoms with similar electronegativities will form covalent bonds. Atoms with very different electronegativities will form ionic bonds. And atoms with intermediate electronegativities will form metallic bonds.
Physical Properties of Glass
The unique atomic arrangement of glass significantly influences its physical properties, distinguishing it from other materials.
Glass exhibits exceptional strength due to the random and disordered arrangement of its atoms. This disordered structure prevents the formation of regular crystal planes, making it difficult for cracks to propagate through the material.
Transparency
The transparency of glass is attributed to its amorphous structure. The absence of long-range atomic order eliminates the scattering of light waves, allowing light to pass through the material with minimal distortion.
Thermal Stability
Glass possesses high thermal stability, making it resistant to thermal shock. The lack of regular atomic arrangements prevents the formation of defects that could lead to thermal expansion or contraction. This property makes glass suitable for applications involving temperature fluctuations, such as cookware and laboratory glassware.
Comparison with Other Materials
- Compared to metals, glass has lower strength but higher transparency and thermal stability.
- In contrast to ceramics, glass is amorphous, lacks a regular crystalline structure, and has a lower melting point.
Manufacturing Processes of Glass
Glass manufacturing involves a series of processes that transform raw materials into the final glass product. These processes influence the atomic arrangement and properties of the glass.
Melting, Atomic arrangement of a glass pitcher
The initial step in glass production is melting the raw materials, typically a mixture of silica sand, soda ash, and limestone. These components are heated to extremely high temperatures in a furnace, causing them to fuse and form a molten glass.
Molding
Once the glass is molten, it is shaped into the desired form through various molding techniques. These techniques include:
- Blowing:Molten glass is blown into a mold using a blowpipe, creating hollow shapes.
- Casting:Molten glass is poured into a mold and allowed to cool and solidify.
- Pressing:Molten glass is pressed between two molds to form flat or curved shapes.
Annealing
After molding, the glass undergoes a controlled cooling process called annealing. This process involves gradually cooling the glass over a period of time, which relieves internal stresses and prevents the glass from shattering.
Applications of Glass: Atomic Arrangement Of A Glass Pitcher
Glass, with its unique atomic arrangement, finds widespread applications in various industries due to its exceptional properties. Its transparency, strength, and resistance to heat and chemicals make it a versatile material for diverse uses.
Construction
- Windows: Glass allows natural light to enter buildings while providing protection from the elements.
- Glass Facades: Glass facades enhance building aesthetics and provide energy efficiency by regulating heat and light.
- Glass Skylights: Skylights made of glass maximize natural daylighting, reducing energy consumption.
Optics
- Lenses: Glass is used in lenses for cameras, telescopes, and other optical instruments due to its ability to refract light.
- Optical Fibers: Glass fibers transmit light signals over long distances, enabling high-speed data transmission.
- Prisms: Glass prisms are used to disperse light into its constituent colors, enabling applications in spectroscopy and color separation.
Electronics
- Glass Substrates: Glass substrates provide a smooth and chemically inert surface for the deposition of electronic components.
- Liquid Crystal Displays (LCDs): Glass is used as the substrate and cover plate for LCDs, providing a clear and durable display surface.
- Semiconductors: Glass is employed as a dielectric material in semiconductor devices, isolating different components and preventing electrical leakage.
Clarifying Questions
What distinguishes the atomic arrangement of glass from crystalline materials?
Unlike crystalline materials with their highly ordered atomic structure, glass exhibits a unique amorphous arrangement where atoms are randomly distributed, lacking the long-range order found in crystals.
How do different types of bonds contribute to the cohesion of atoms in glass?
Covalent bonds, ionic bonds, and van der Waals forces collectively hold atoms together in glass. Covalent bonds, the strongest among them, involve the sharing of electrons between atoms, while ionic bonds result from the attraction between oppositely charged ions. Van der Waals forces, weaker than covalent and ionic bonds, arise from temporary fluctuations in electron distribution.
In what ways does the atomic arrangement influence the physical properties of glass?
The random atomic arrangement of glass contributes to its isotropic nature, meaning its properties are the same in all directions. This arrangement also imparts strength, transparency, and thermal stability to glass, making it suitable for a wide range of applications.